Happy Thursday!
Today, I want to address a fantastic and surprisingly common question that landed in my inbox this week:
Hey Hunter, I’ve been researching peptides and keep seeing warnings about endotoxins and LPS. How do I know if the peptides I’m using are safe, and what kind of testing should I look for?
This is one of those rabbit holes that can either keep you up at night or empower you to make smart, informed decisions about your health.
So today I’m going to break it all down for you, including what endotoxins and LPS really are, why they matter for peptides, how reputable labs test for them, and what you should actually look for in a Certificate of Analysis (COA).
By the end, you’ll know exactly how to protect yourself and why companies like BioLongevity Labs take this so seriously.
On September 13-14, Jay and I will be speaking at the Health Optimisation Summit in London, UK on the subject of peptides.
If you buy a ticket before July 12, you can take advantage of early bird pricing. The general admission ticket is £206.10 (~$240) when you use code HUNTER at checkout.
I attended the Austin, TX summit earlier this year and it is WELL worth the ticket price.
What Are Endotoxins and LPS?
Endotoxins are toxic components found in the outer membrane of certain bacteria, especially gram-negative bacteria.
The main culprit?
Lipopolysaccharide (LPS).
Think of LPS as the bacteria’s armor.
When bacteria break apart (even after they’re dead), this LPS gets released into their surroundings, including water, lab equipment, and even supposedly “pure” pharmaceutical products.
Why should you care?
Because even a tiny amount of LPS injected or infused into your body can cause a massive immune response, such as fever, chills, headaches, and at high doses, potentially dangerous, life-threatening reactions.
The human immune system is incredibly sensitive to LPS.
It sees it as a fire alarm that says: “Intruder! Bacterial attack!”
This is why it’s called a “pyrogen,” aka something that causes fever.
For peptide enthusiasts, athletes, or anyone exploring advanced therapeutics, knowing your peptides are free of endotoxins is non-negotiable.
The presence of LPS can literally turn a healing experiment into an inflammatory disaster.
Why Endotoxins Matter for Research Peptides
Whether a peptide is manufactured through bacterial fermentation or chemical synthesis, contamination can occur at multiple stages, including the use of raw materials, water, equipment, and even the air in the facility.
That means endotoxins can sneak into even the most pristine-looking vial.
This matters for two big reasons:
Safety: Injecting endotoxins can provoke immediate immune reactions, from mild fevers to full-blown shock in severe cases. In research settings, animals or cells exposed to LPS-contaminated peptides can show false results, meaning you might blame or credit the peptide for effects actually caused by LPS.
Data Integrity: Endotoxins can totally confound your research. If you’re tracking the anti-inflammatory effects of BPC-157, TB-500, GLP-1s, or even mitochondrial peptides like SS-31, the last thing you want is the real effect being overshadowed by an immune system freak-out caused by bacterial debris.
This is why every serious peptide lab will not just test for purity and potency, but will have specific numbers on endotoxin/LPS content.
How Endotoxin Testing Works
The industry standard for detecting endotoxins is the Limulus Amebocyte Lysate (LAL) assay.
Today, most labs use a modern version called the chromogenic LAL assay, which measures color change in response to endotoxins and is sensitive down to as little as 0.01 EU/mL (Endotoxin Units per milliliter).
Big pharma and the FDA set strict thresholds.
Most injectable drugs must have less than 0.25–0.5 EU/mL to be considered safe.
For context, 1 EU is a staggeringly small amount, and the average pharmaceutical product is expected to be well below these levels.
A Real Example
Here’s an example from a real certificate of analysis for BioLongevity Labs’ SS-31 peptide.
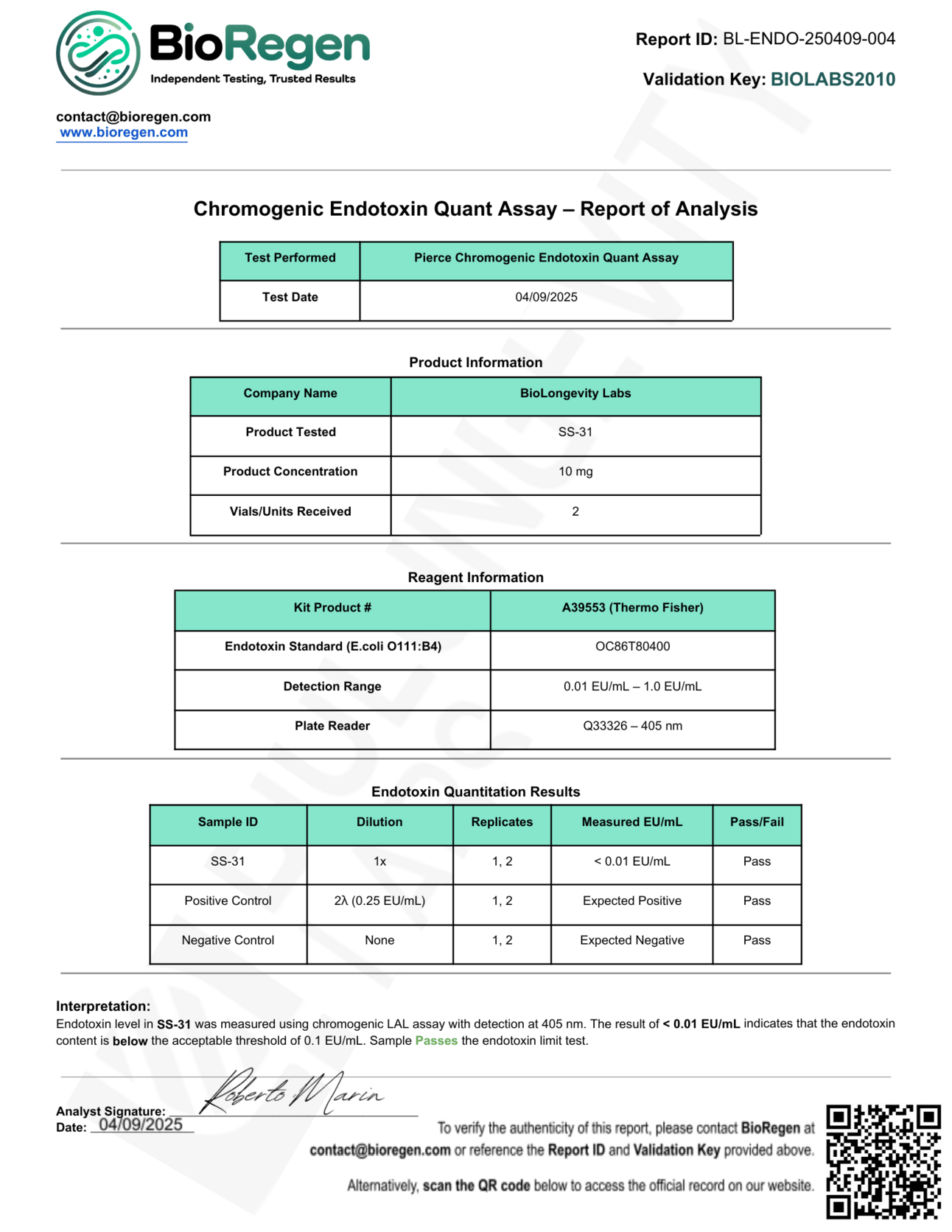
We sent samples of our 10mg SS-31 to BioRegen, an independent testing lab, for a full chromogenic endotoxin quant assay. The results were:
Measured Endotoxin Content: <0.01 EU/mL
Threshold for Safety: 0.1 EU/mL (10x higher than our result)
Test Used: Pierce Chromogenic Endotoxin Quant Assay (Thermo Fisher)
Controls: Both positive (spiked with known endotoxin) and negative (no endotoxin) controls performed as expected, confirming the test’s accuracy.
Result: PASS
What does this mean for you?
It means the endotoxin content in this batch of SS-31 is an order of magnitude below the accepted safety threshold.
In plain English: this is as safe and “clean” as you can get when it comes to injectable peptides.
Every batch is not only tested for purity and potency but also for microbial safety, so you can feel confident about what’s going into your body.
What Happens When Peptides Aren’t Tested?
I want to highlight a recent peer-reviewed study published in JMIR 2024 that turned the spotlight on compounded and gray-market peptide products.
Researchers bought 25 different samples of semaglutide from U.S. and international online vendors.
What did they find?
All samples contained measurable endotoxins, ranging from 2.16 to 8.95 EU/mg—far above what you’d see in a regulated product.
Many vials contained far less actual peptide than advertised, with some vials having only a fraction of the intended dose.
The endotoxin contamination levels were high enough to potentially trigger fever, inflammation, or worse, putting users at real risk.
This is exactly why third-party endotoxin testing and a legitimate COA are absolutely critical when purchasing research peptides.
What You Should Look For
So, what’s the takeaway?
First, DON’T be afraid.
Most peptide companies are in the business for the right reasons and are doing their best to verify that all of their products are sterile.
Nonetheless, whenever you’re considering a peptide, demand to see the Certificate of Analysis.
Here’s your checklist:
Does the COA include an endotoxin test? Look for terms like “LAL assay,” “chromogenic endotoxin quant assay,” or an explicit number for EU/mL or EU/mg.
Is the result well below the accepted safety threshold? (Ideally <0.1 EU/mL, often much lower in high-quality labs.)
Was the test performed by an independent, reputable lab?
Is the result recent and batch-specific? Outdated or generic results are red flags.
Bottom line: If the vendor can’t or won’t provide a real COA, run the other way.
But even with a COA, it’s never a bad idea to double-check, especially if you’re getting unexpected reactions or if you just want the peace of mind of third-party confirmation.
Independent labs will often run an endotoxin test for a reasonable fee.
Final Thoughts
Look, the world of therapeutic peptides is both thrilling and complex.
The best way to protect yourself is through knowledge and transparency.
Endotoxins and LPS aren’t something you should lose sleep over, but as Ronald Reagan once said, “trust, but verify.”
At BioLongevity Labs, we run every batch through rigorous, third-party endotoxin testing and share those results with you.
So next time you hear someone dismissing the importance of endotoxin testing, send them this email.
Because when it comes to your health, there’s no such thing as being “too careful.”
Best
Hunter Williams
